Phosphorus-Mo2C@carbon nanowires toward efficient electrochemical hydrogen evolution: composition, structural and electronic regulation†
Abstract
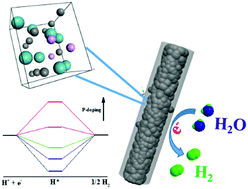
To explore high-performance electrocatalysts, electronic regulation on active sites is essentially demanded. Herein, we propose controlled phosphorus doping to effectively modify the electronic configuration of nanostructured Mo2C, accomplishing a benchmark performance of noble-metal-free electrocatalysts in the hydrogen evolution reaction (HER). Employing MoOx–phytic acid–polyaniline hybrids with tunable composition as precursors, a series of hierarchical nanowires composed of phosphorus-doped Mo2C nanoparticles evenly integrated within conducting carbon (denoted as P-Mo2C@C) are successfully obtained via facile pyrolysis under inert flow. Remarkably, P-doping into Mo2C can increase the electron density around the Fermi level of Mo2C, leading to weakened Mo–H bonding toward promoted HER kinetics. Further density functional theory calculations show that the negative hydrogen-binding free energy (ΔGH*) on pristine Mo2C gradually increases with P-doping due to electron transfer and steric hindrance by P on the Mo2C surface, indicating the effectively weakened strength of Mo–H. With optimal doping, a ΔGH* approaching 0 eV suggests a good balance between the Volmer and Heyrovsky/Tafel steps in HER kinetics. As expected, the P-Mo2C@C nanowires with controlled P-doping (P: 2.9 wt%) deliver a low overpotential of 89 mV at a current density of −10 mA cm−2 and striking kinetic metrics (onset overpotential: 35 mV, Tafel slope: 42 mV dec−1) in acidic electrolytes, outperforming most of the current noble-metal-free electrocatalysts. Elucidating feasible electronic regulation and the remarkably enhanced catalysis associated with controlled P-doping, our work will pave the way for developing efficient noble-metal-free catalysts via rational surface engineering.


 Please wait while we load your content...
Please wait while we load your content...