Electrolysis of liquid ammonia for hydrogen generation†
Abstract
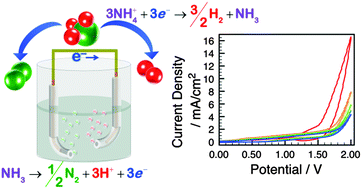
One route to efficient storage, transportation and utilization of renewable hydrogen is through its conversion into ammonia (NH3). In order to examine the feasibility of an NH3 fuel cycle, the electrolysis of NH3 – both as a liquid and dissolved in N,N-dimethylformamide – was investigated using platinum electrodes. The current scaled with electrolyte concentration, but was nominally independent of composition, suggesting solution resistance limitations. NH3 was found to be the chemical species oxidized at the anode which produced N2, but also resulted in a poisoned electrode surface which introduced an additional overpotential of ∼0.5 V. Surprisingly, NH4+ was the species reduced at the cathode via a one-electron transfer process to form 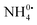 , prior to H2 generation, which resulted in an additional cathodic overpotential. In addition to establishing the two half reactions of liquid ammonia electrolysis, the formal potentials of the reactions and the kinetic overpotentials were quantified.
, prior to H2 generation, which resulted in an additional cathodic overpotential. In addition to establishing the two half reactions of liquid ammonia electrolysis, the formal potentials of the reactions and the kinetic overpotentials were quantified.

 Please wait while we load your content...
Please wait while we load your content...