Oligomeric-Schiff bases as negative electrodes for sodium ion batteries: unveiling the nature of their active redox centers†
Abstract
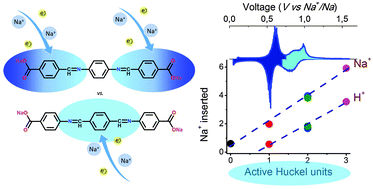
Oligomeric Schiff bases with carboxylate end-groups are crystalline, electrochemically active materials at voltages below 1.2 V vs. Na+/Na. This low redox voltage along with delivered capacities up to 340 mA h g−1 makes them potential anode materials for sodium ion batteries. The electrochemical performance is optimized by maximizing the number of active units with respect to the total chain length. We report for the first time the electrochemical activity of the 10-π-electron end group (–−OOC–ϕ–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) (ϕ refers to the phenyl group) and central (–N
N–) (ϕ refers to the phenyl group) and central (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–ϕ–C
C–ϕ–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) Hückel units. This enhanced activity is due to the larger stability of planar molecular regions with respect to out-of-plane inactive (–−OOC–ϕ–N
N–) Hückel units. This enhanced activity is due to the larger stability of planar molecular regions with respect to out-of-plane inactive (–−OOC–ϕ–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and (–C
C–) and (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–ϕ–N
N–ϕ–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), as confirmed by DFT calculations.
C–), as confirmed by DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...