Electrochemical tuning of olivine-type lithium transition-metal phosphates as efficient water oxidation catalysts†
Abstract
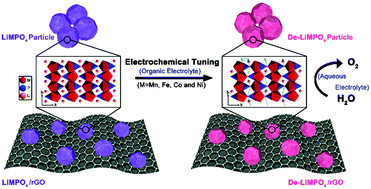
The oxygen evolution reaction is of paramount importance in clean energy generation and storage. While the common approach in search of active, durable and cost-effective oxygen evolution catalysts involves the development of novel materials, it is equally important to tune the properties of existing materials so as to improve their catalytic performance. Here, we demonstrate the general efficacy of electrochemical lithium tuning in organic electrolyte on enhancing the oxygen evolution catalytic activity of olivine-type lithium transition metal phosphates, a widely-researched family of cathode materials in lithium ion batteries. By continuously extracting lithium ions out of lithium transition metal phosphates, the materials exhibited significantly enhanced water oxidation catalytic activity. Particularly, the electrochemically delithiated Li(Ni,Fe)PO4 nanoparticles anchored on reduced graphene oxide sheets afforded outstanding performance, generating a current density of 10 mA cm−2 at an overpotential of only 0.27 V for over 24 h without degradation in 0.1 M KOH, outperforming the commercial precious metal Ir catalysts.

 Please wait while we load your content...
Please wait while we load your content...