Positive onset potential and stability of Cu2O-based photocathodes in water splitting by atomic layer deposition of a Ga2O3 buffer layer†
Abstract
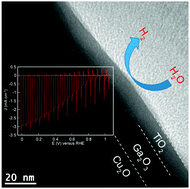
The Cu2O-based photocathode is considered as one of the most promising photocathodes for high performance water splitting under sunlight. However, the relatively negative onset potential for H2 production of these photocathodes impedes further optimization of the solar-to-fuel conversion efficiency. Here, a thin Ga2O3 buffer layer is introduced between the Cu2O absorber layer and the TiO2 protective layer by atomic layer deposition to increase the photovoltage. For the optimized TiO2 deposition temperature, the Pt/TiO2/Ga2O3/Cu2O electrode achieves a high cathodic photocurrent of −2.95 mA cm−2 at 0 V vs. RHE and an extremely positive onset potential of 1.02 V vs. RHE (defined as the potential where photocathodic current reaches 20 μA cm−2 under air-mass 1.5 global illumination), benefiting from a buried p–n junction and a favorable band alignment. The Pt/TiO2/Ga2O3/Cu2O electrodes exhibit a stable cathodic current for 2 h under continuous illumination of a 500 W Xe lamp for the TiO2 deposition temperatures below 180 °C.

 Please wait while we load your content...
Please wait while we load your content...