A membrane-less electrolyzer for hydrogen production across the pH scale†
Abstract
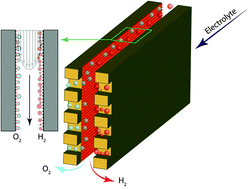
The development of deployable water-splitting devices is hindered by the cost of electricity and the lack of stable ion conducting membranes that can operate across the pH scale, impose low ionic resistances and avoid product mixing. The membrane-less approach developed in this work breaks this paradigm and demonstrates for the first time an electrolyzer capable of operating with lower ionic resistance than benchmark membrane-based electrolyzers using virtually any electrolyte. Our method separates product gases by controlling the delicate balance between fluid mechanic forces in the device. The devices presented here are able to split water at current densities over 300 mA cm−2, with more than 42% power conversion efficiency, and crossover of hydrogen gas into the oxidation side as low as 0.4%, leading to a non-flammable and continuous hydrogen fuel stream. Furthermore, ability to use buffered electrolytes allows for the incorporation of earth-abundant catalysts that can only operate at moderate to high pH values.

 Please wait while we load your content...
Please wait while we load your content...