Bio-inspired organic cobalt(ii) phosphonates toward water oxidation†
Abstract
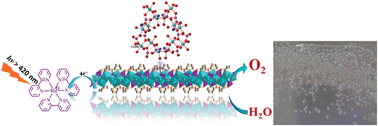
The development of artificial photosynthesis systems that can efficiently catalyze water oxidation to generate oxygen remains one of the most important challenges in solar energy conversion to chemical energy. In photosystem II (PSII), the Mn4CaO5 cluster adopts a distorted coordination geometry and every two octahedra are linked by di-μ-oxo (edge-shared) or mono-μ-oxo (corner-shared) bridges, which is recognized as a critical structure motif for catalytic water oxidation. These structural features provide guidance on the design and synthesis of new water oxidation catalysts. Herein we synthesized a new layered organic cobalt phosphonate crystal, Co3(O3PCH2–NC4H7–CO2)2·4H2O (1) and demonstrate it as a heterogeneous catalyst for water oxidation. Its catalytic activity was compared to those of cobalt phosphonates with different structures (2–4) in terms of O2 evolution rate and O2 yield under the same reaction conditions. The compound with both mono- and di-μ-oxo bridged octahedral cobalt displays superior catalytic activity. In contrast, the presence of only mono-μ-oxo bridged cobalt in the structure results in lower O2 yield and O2 evolution rate. Further structural analysis reveals that the presence of a longer Co–N bond induces a distorted dissymmetry coordination geometry, and consequently facilitates water oxidation. These results provide important insight into the design of water oxidation catalysts.

 Please wait while we load your content...
Please wait while we load your content...