Low bandgap conjugated polymers based on mono-fluorinated isoindigo for efficient bulk heterojunction polymer solar cells processed with non-chlorinated solvents†
Abstract
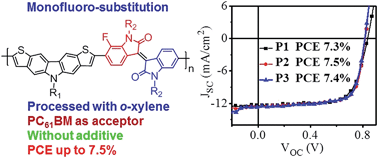
Three low bandgap conjugated polymers based on 7-fluorinated isoindigo (IID1F) and dithieno[3,2-b;6,7-b]carbazole (DTC), i.e., poly[N-dodecyldithieno[3,2-b;6,7-b]carbazole-alt-7-fluoro-N,N-di(2-octyldodecyl)isoindigo] (P1), poly[N-dodecyldithieno[3,2-b;6,7-b]carbazole-alt-7-fluoro-N,N-di(3-octyltridecyl)isoindigo] (P2) and poly[N-dodecyldithieno[3,2-b;6,7-b]carbazole-alt-7-fluoro-N,N-di(4-octyltetradecyl)isoindigo] (P3), were synthesized. All three polymers are soluble in non-chlorinated solvent o-xylene owing to regio-random distribution of F-atoms along the conjugated backbone. The position of the alkyl-branching point has a negligible influence on energy levels and absorption spectra of the polymers, but has a small effect on their charge transport properties. Bulk heterojunction (BHJ) polymer solar cells (PSCs) of the polymers were fabricated with phenyl-C61-butyric acid methyl ester (PC61BM) as an electron acceptor. When o-xylene was used as a solvent, all three polymers delivered power conversion efficiencies (PCEs) above 7%. P2 exhibited the best device performance with a PCE of 7.5%. The devices processed with o-xylene showed higher device efficiency than those fabricated with o-dichlorobenzene (o-DCB) since the films of polymer:PC61BM blends prepared with o-xylene exhibited better morphology and higher and more balanced charge-carrier mobilities, leading to less recombination loss and higher fill factor (FF).

 Please wait while we load your content...
Please wait while we load your content...