Abstract
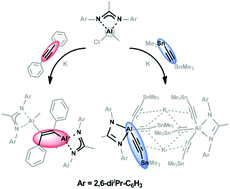
Two ethylene-bridged methylaluminium amidinates and one aluminium amidinate containing three terminal trimethylstannyl-ethynyl groups interconnected by π-coordinated potassium ions were prepared in situ. The re-oxidation of the ethylene-bridged compound by iodine followed by further reduction using the same activation procedure demonstrated the versatility of the approach. The reactivity of an ethylene-bridged methylaluminum amidinate towards HCl was examined to demonstrate the building block concept. DFT calculations were performed to gain insight into the mechanism of the in situ activation of diphenylacetylene.

 Please wait while we load your content...
Please wait while we load your content...