Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes†
Abstract
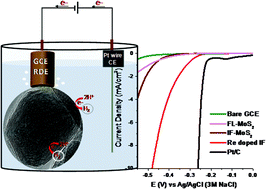
Electrochemical generation of hydrogen by non-precious metal electrocatalysts at a lower overpotential is a focus area of research directed towards sustainable energy. The exorbitant costs associated with Pt-based catalysts is the major bottleneck associated with commercial-scale hydrogen generation. Strategies for the synthesis of cost-effective and stable catalysts are thus key for a prospective ‘hydrogen economy’. In this report, we highlight a novel and general strategy to enhance the electrochemical activity of molybdenum disulfide (MoS2) in a fullerene structure (IF-). In particular, pristine (undoped) and rhenium-doped nanoparticles of MoS2 with fullerene-like structures (IF-MoS2) were studied, and their performance as catalysts for the hydrogen evolution reaction (HER) was compared to that of 2H-MoS2 particles (platelets). The current density of the IF-MoS2 was higher by one order of magnitude than that of few-layer (FL-) MoS2, due to the enhanced density of the edge sites. Furthermore, Re doping of as low as 100 ppm in IF-MoS2 decreased the onset potential by 60–80 mV and increased the activity by 60 times compared with that of the FL-MoS2. The combined synergistic effect of Re doping and the IF structure not only changes the intrinsic nature of the MoS2 but also increases its reactivity. This strategy highlights the potential use of the IF structure and Re doping in electrocatalytic hydrogen evolution using MoS2-based catalysts.

 Please wait while we load your content...
Please wait while we load your content...