Nitrogen–boron coordination versus OH⋯N hydrogen bonding in pyridoxaboroles – aza analogues of benzoxaboroles†
Abstract
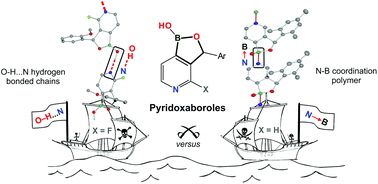
Pyridoxaboroles – fused heterocyclic systems composed of pyridine and five-membered oxaborole rings – have been obtained for the first time from simple halopyridines. Thus, 6-butyl-2-(3′-bromo-4′-pyridyl)-(N–B)-1,3,6,2-dioxazaborocan obtained from 3-bromopyridine was converted into a lithio derivative by Br/Li exchange using nBuLi/THF at −85 °C. This intermediate was trapped with benzaldehydes to give the corresponding pyridoxaboroles after hydrolysis. The use of chlorodiphenylsilane as an electrophile gave rise to a related pyridosiloxaborole. The fluorinated pyridoxaborole was obtained by deprotonation of α-(2-methoxyphenyl)-2-fluoro-4-iodopyridylmethanol with NaH and consecutive iodine–lithium exchange/boronation followed by hydrolysis. Single-crystal X-ray analysis of pyridino[4,3-c]-1,3-dihydro-1-hydroxy-3-mesityl[2,1]oxaborole revealed the formation of a unique 1D coordination polymer based on N–B dative bonds between monomeric molecules. In contrast, the crystal structure of 2-fluoropyridino[4,3-c]-1,3-dihydro-1-hydroxy-3-(2′-methoxyphenyl)[2,1]oxaborole features an infinite H-bonded chain as the main structural motif. The presented considerations are quantified in terms of various computational methods (single molecule and dimer energy calculations, electron density topology, NBO analyses) providing a comprehensive picture of the structural properties of pyridoxaboroles.

 Please wait while we load your content...
Please wait while we load your content...