Synthesis, characterization and antibacterial behavior of water-soluble carbosilane dendrons containing ferrocene at the focal point†
Abstract
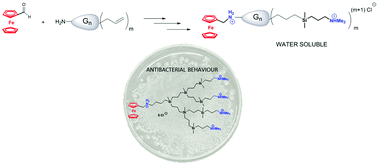
A series of novel water-soluble ammonium-terminated carbosilane dendrons containing a ferrocene unit at the focal point were synthesized, in order to combine the unique redox activity of ferrocene and the precisely designed structure of the dendrons with the aim to evaluate them as a new class of potential organometallic-based antibacterial compounds. The synthetic route is based on the initial amination of ferrocenecarboxaldehyde with carbosilane dendrons that contain allyl groups on the surface followed by reduction of the in situ prepared imine product, and the subsequent functionalization of the periphery with terminal amine groups by hydrosilylation reactions. Systems quaternized with HCl are soluble and stable in water or other protic solvents. The obtained compounds were spectrally and electrochemically (cyclic voltammetry) characterized, and diffusion-ordered spectroscopy experiments were conducted to determine the size of the dendritic wedges in solution. The antibacterial activity of these compounds was evaluated using Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli), which shows that the first and second generations of cationic dendrons are broad spectrum antibacterial agents, i.e. selective and effective in both bacterial strains.

 Please wait while we load your content...
Please wait while we load your content...