Dichlorodioxomolybdenum(vi) complexes bearing oxygen-donor ligands as olefin epoxidation catalysts†
Abstract
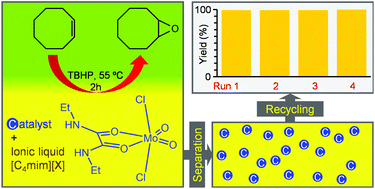
Treatment of the solvent adduct [MoO2Cl2(THF)2] with either 2 equivalents of N,N-dimethylbenzamide (DMB) or 1 equivalent of N,N′-diethyloxamide (DEO) gave the dioxomolybdenum(VI) complexes [MoO2Cl2(DMB)2] (1) and [MoO2Cl2(DEO)] (2). The molecular structures of 1 and 2 were determined by single-crystal X-ray diffraction. Both complexes present a distorted octahedral geometry and adopt the cis-oxo, trans-Cl, cis-L configuration typical of complexes of the type [MoO2X2(L)n], with either the monodentate DMB or bidentate DEO oxygen-donor ligands occupying the equatorial positions trans to the oxo groups. The complexes were applied as homogeneous catalysts for the epoxidation of olefins, namely cis-cyclooctene (Cy), 1-octene, trans-2-octene, α-pinene and (R)-(+)-limonene, using tert-butylhydroperoxide (TBHP) as oxidant. In the epoxidation of Cy at 55 °C, the desired epoxide was the only product and turnover frequencies in the range of ca. 3150–3200 mol molMo−1 h−1 could be reached. The catalytic production of cyclooctene oxide was investigated in detail, varying either the reaction temperature or the cosolvent. Complexes 1 and 2 were also applied in liquid–liquid biphasic catalytic epoxidation reactions by using an ionic liquid of the type [C4mim][X] (C4mim = 1-n-butyl-3-methylimidazolium; X = NTf2, BF4 or PF6] as a solvent to immobilise the metal catalysts. Recycling for multiple catalytic runs was achieved without loss of activity.


 Please wait while we load your content...
Please wait while we load your content...