Synthesis and unique reversible splitting of 14-membered cyclic aminomethylphosphines on to 7-membered heterocycles†
Abstract
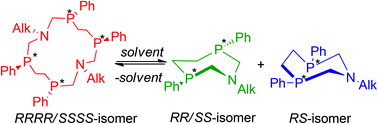
A novel type of 14-membered cyclic polyphosphine, namely 1,8-diaza-3,6,10,13-tetraphosphacyclotetradecanes 2a–4a has been synthesized by the condensation of 1,2-bis(phenylphosphino)ethane, formaldehyde and alkylamines (isopropylamine, ethylamine and cyclohexylamine) as a RRRR/SSSS-stereoisomer. The structure of macrocycle 2a was investigated by NMR-spectroscopy and X-ray crystal structure analysis. The unique reversible processes of macrocycles 2a–4a splitting onto the corresponding rac- (2b–4b) and meso- (2c–4c) stereoisomers of 1-aza-3,6-diphosphacycloheptanes were discovered.

 Please wait while we load your content...
Please wait while we load your content...