Ligand field influence on the electronic and magnetic properties of quasi-linear two-coordinate iron(ii) complexes†‡
Abstract
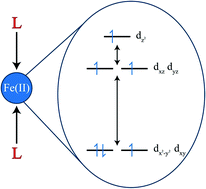
The 2 to 300 K magnetic susceptibilities of Fe{N(SiMe2Ph)2}2, 1, Fe{N(SiMePh2)2}2, 2, and the diaryl complex Fe(ArPri4)2, 3, where ArPri4 is C6H3-2,6(C6H3-2,6-Pri2)2 have been measured. Initial fits of these properties in the absence of an independent knowledge of their ligand field splitting have proven problematic. Ab initio calculations of the CASSCF/RASSI/SINGLE-ANISO type have indicated that the orbital energies of the complexes, as well as those of Fe(ArMe6)2, 4, where ArMe6 is C6H3-2,6(C6H2-2,4,6-Me3)2), are in the order dxy ≈ dx2−y2 < dxz ≈ dyz < dz2, and the iron(II) complexes in this ligand field have the (dxy, dx2−y2)3(dxz, dyz)2(dz2)1 ground electronic configuration with a substantial orbital contribution to their effective magnetic moments. An ab initio-derived ligand field and spin–orbit model is found to yield an excellent simulation of the observed magnetic properties of 1–3. The calculated ligand field strengths of these ligands are placed in the broader context of common coordination ligands in hypothetical two-coordinate linear iron(II) complexes. This yields the ordering I− < H− < Br− ≈ PMe3 < CH3− < Cl− ≈ C(SiMe3)3− < CN− ≈ SArPri6− < ArPri4− < ArMe6− ≈ N3− < NCS− ≈ NCSe− ≈ NCBH3− ≈ MeCN ≈ H2O ≈ NH3 < NO3− ≈ THF ≈ CO ≈ N(SiMe2Ph)2− ≈ N(SiMePh2)2− < F− ≈ N(H)ArPri6− ≈ N(SiMe3)Dipp− < OArPri4−. The magnetic susceptibility of the bridged dimer, [Fe{N(SiMe3)2}2]2, 5, has also been measured between 2 and 300 K and a fit of χMT with the isotropic Heisenberg Hamiltonian, Ĥ = −2JŜ1·Ŝ2 yields an antiferromagnetic exchange coupling constant, J, of −131(2) cm−1.
- This article is part of the themed collection: In memory of Professor Kenneth Wade


 Please wait while we load your content...
Please wait while we load your content...