16-Electron pentadienyl- and cyclopentadienyl-ruthenium half-sandwich complexes with bis(imidazol-2-imine) ligands and their use in catalytic transfer hydrogenation†
Abstract
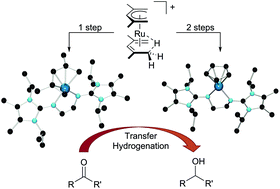
Bis(η5-2,4-dimethylpentadienyl)ruthenium(II), [(η5-C7H11)2Ru] (1, “open ruthenocene”), which has become accessible in high yield and large quantities via an isoprene-derived diallyl ruthenium(IV) complex, can be converted into the protonated open ruthenocene 2 by treatment with HBF4 and subsequently into the protonated half-open ruthenocene 3 by reaction with cyclopentadiene. The electronic structure of 3 was studied by DFT methods, revealing that the CH-agostic complex [(η5-C5H5)Ru{(1-4η)-C7H12-η2-C5,H5}]BF4 (A) represents the global minimum, which is 3.7 kcal mol−1 lower in energy than the hydride complex [(η5-C5H5)RuH(η5-C7H11)]BF4 (B). 2 and 3 were treated with the ligands N,N′-bis(1,3,4,5-tetramethylimidazolin-2-ylidene)-1,2-ethanediamine (BLMe) and N,N′-bis(1,3-diisopropyl-4,5-dimethylimidazolin-2-ylidene)-1,2-ethanediamine (BLiPr) to afford the cationic 16-electron pentadienyl and cyclopentadienyl complexes [(η5-C7H11)Ru(BLR)]BF4 (4a, R = Me; 4b, R = iPr) and [(η5-C5H5)Ru(BLR)]BF4 (5a, R = Me; 5b, R = iPr). All complexes catalyse the transfer hydrogenation of acetophenone in isopropanol, and the most active complex 4a in this reaction was employed for the hydrogenation of a broader range of aliphatic and aromatic ketones.

 Please wait while we load your content...
Please wait while we load your content...