Insertion, elimination and isomerisation of olefins at alkylaluminium hydride: an experimental and theoretical study†
Abstract
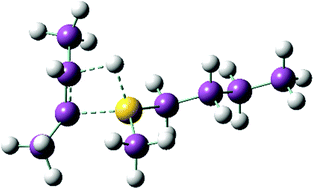
The insertion, elimination and isomerisation of octenes with di-n-octylaluminium hydride [HAl(Oct)2], tri-n-octylaluminium [Al(Oct)3] and sec-octylaluminium species have been studied as individual steps in a putative aluminium based contrathermodynamic olefin isomerisation process. While elimination of 1-octene from [Al(Oct)3] is energetically unfavourable, the process is driven by high temperature vacuum distillation, leading to very high selectivity to 1-octene (>97%). At high conversions the [HAl(Oct)2] so obtained exists predominately as hydride-bridged cyclic oligomers, whereas at low conversion the mixed alkyl/hydride-bridged dimer [(Oct)2Al(μ-H)(μ-Oct)Al(Oct)2] is the major species. Di-n-octylaluminium hydride recovered after olefin elimination may be recycled and is active toward re-insertion of octenes. Internal octenes (cis- and trans-2-, 3- and 4-octene) only partially insert however, and even after prolonged heating there is no significant secondary to primary alkyl isomerisation evident.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...