Reduction of hydroxy-functionalised carbaboranyl carboxylic acids and ketones by organolithium reagents†
Abstract
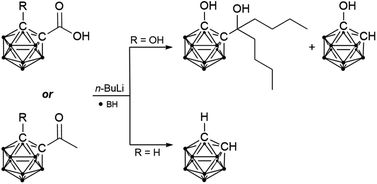
While the reaction of carbaboranyl carboxylic acids and ketones with organolithium reagents generally leads to cleavage of the exo-polyhedral C–C bond, introduction of a hydroxyl group at the second carbon atom of the cluster enables the reduction of the carbonyl compounds to tertiary alcohols. The proposed mechanism involving the formation of dimeric contact ion pairs was supported by X-ray crystallography and theoretical calculations.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...