Amphiphilic zinc phthalocyanine photosensitizers: synthesis, photophysicochemical properties and in vitro studies for photodynamic therapy†
Abstract
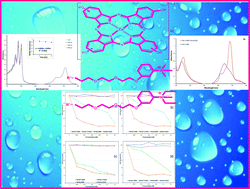
Peripherally and non-peripherally tetra-substituted zinc(II) phthalocyanines bearing 2-(2-{2-[3-(dimethylamino)phenoxy]ethoxy}ethoxy)ethoxy and 2-(2-{2-[3-(diethylamino)phenoxy]ethoxy}ethoxy)ethoxy groups (2a, 5a, 3a and 6a) were synthesized by cyclotetramerization of the corresponding phthalonitriles (2, 5, 3 and 6). Their quaternized ionic derivatives (2b, 5b, 3b and 6b) were also synthesized by the reaction of them with methyl iodide. The novel compounds were characterized by using standard spectroscopic techniques such as FT-IR, 1H NMR, 13C NMR, UV-vis, mass and elemental analyses. The obtained quaternized phthalocyanines (2b, 5b, 3b and 6b) showed amphiphilic behaviour with excellent solubility in both organic and aqueous solutions, which makes them potential photosensitizers for use in photodynamic therapy (PDT) of cancer. The photophysical (fluorescence quantum yields and lifetimes) and photochemical (singlet oxygen and photodegradation quantum yields) properties of these novel phthalocyanines were studied in DMSO for both non-ionic and ionic quaternized derivatives. However, these properties were examined in both DMSO and phosphate buffer solution (PBS) for quaternized ionic phthalocyanines. The effects of the positions of substituents (peripheral or non-peripheral) and the quaternization of the nitrogen atoms on the substituents about their photophysical and photochemical properties were also compared in this study. The bovine serum albumin (BSA) binding behaviours of the studied quaternized ionic zinc(II) phthalocyanines were also described in PBS solutions. The quaternized phthalocyanines (2b, 5b, 3b and 6b) successfully displayed light-dependent photodamage in HeLa and HuH-7 cancer cells in photodynamic therapy treatment. The photosensitivity and the intensity of damage were found directly related to the concentration of the photosensitizers.

 Please wait while we load your content...
Please wait while we load your content...