Influence of substituents on cation–anion contacts in imidazolium perrhenates†
Abstract
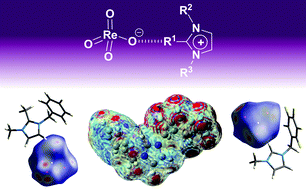
A series of imidazolium perrhenates with different substituents at the imidazolium ring were synthesised and characterised, including single crystal X-ray diffraction. The effect of the substitution pattern on the state of aggregation of the compounds, the charge delocalisation and the ion pairing interaction via hydrogen bonds was studied. Particularly the substitution at the C2 position of the imidazolium ring was shown to be crucial to fine-tune the ion contacts. Fluorinated substituents appear to exhibit enhanced interionic interactions. The ability to tune the degree of contacts of the perrhenate anion allows for adjusting the nucleophilicity of this anion.

 Please wait while we load your content...
Please wait while we load your content...