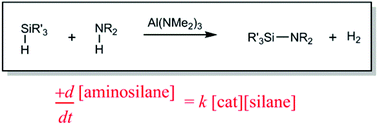
Abstract
The aluminium amide Al(NMe2)3 acts as a stoichiometric or catalytic reagent in dehydrogenic Si–N bond formation using amines and silanes. Although of limited substrate scope, this represents the first p-block metal catalytic system for N–H/Si–H dehydrocoupling. The observed catalytic rate law for the formation of aminosilane products in a model study of one of the catalytic reactions suggests a mechanism involving the silane component in the deprotonation of the amine (possibly in the form of a hypervalent silicon hydride).
- This article is part of the themed collections: In memory of Professor Kenneth Wade and Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...