Anion complexation with cyanobenzoyl substituted first and second generation tripodal amide receptors: crystal structure and solution studies†
Abstract
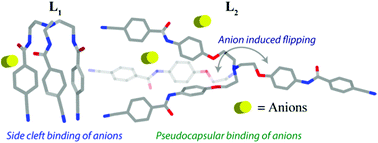
Anion complexation properties of two new tripodal amide receptors have been extensively studied here. Two tripodal receptors have been synthesized from the reaction of cyanobenzoyl acid chloride with two tri-amine building blocks such as (i) tris(2-aminoethyl)amine and (ii) tris(2-(4-aminophenoxy)ethyl)amine, which resulted in the first (L1) and second (L2) generation tripodal amides respectively. A detailed comparison of their coordination behavior with anions is also described by crystallographic and solution state experiments. The crystal structure demonstrates various types of spatial orientations of tripodal arms in two receptors and concomitantly interacts with anions distinctively. Intramolecular H-bonding between amide N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O prevents opening of the receptor cavity in the crystal, which leads to a locked conformation of L1 having C3v symmetry and makes amide hydrogen unavailable for the anion which results in side cleft anion binding. However, in L2 we conveniently shift the anion binding sites to a distant position which increases cavity size as well as rules out any intramolecular H-bonding between amide N–H and C
O prevents opening of the receptor cavity in the crystal, which leads to a locked conformation of L1 having C3v symmetry and makes amide hydrogen unavailable for the anion which results in side cleft anion binding. However, in L2 we conveniently shift the anion binding sites to a distant position which increases cavity size as well as rules out any intramolecular H-bonding between amide N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The crystal structure shows a different orientation of the arms in L2; it adopts a quasi-planar arrangement with C2v symmetry. In the crystal structure two arms are pointed in the same direction and while extending the contact the third arm is H-bonded with the apical N-atom through a –CN group, making a pseudo capsular cavity where the anion interacts. Most importantly spatial reorientation of the receptor L2 from a C2v symmetry to a folded conformation with a C3v symmetry was observed only in the presence of an octahedral SiF62− anion and forms a sandwich type complex. Receptors L1 and L2 are explored for their solution state anion binding abilities. The substantial changes in chemical shifts were observed for the amide (–NH) and aromatic hydrogen (–CH) (especially for F−), indicating the role of these hydrogens in anion binding. The anion interacts with receptor L2 more strongly than L1 as confirmed by 1H NMR titration upon monitoring the –NH signal.
O. The crystal structure shows a different orientation of the arms in L2; it adopts a quasi-planar arrangement with C2v symmetry. In the crystal structure two arms are pointed in the same direction and while extending the contact the third arm is H-bonded with the apical N-atom through a –CN group, making a pseudo capsular cavity where the anion interacts. Most importantly spatial reorientation of the receptor L2 from a C2v symmetry to a folded conformation with a C3v symmetry was observed only in the presence of an octahedral SiF62− anion and forms a sandwich type complex. Receptors L1 and L2 are explored for their solution state anion binding abilities. The substantial changes in chemical shifts were observed for the amide (–NH) and aromatic hydrogen (–CH) (especially for F−), indicating the role of these hydrogens in anion binding. The anion interacts with receptor L2 more strongly than L1 as confirmed by 1H NMR titration upon monitoring the –NH signal.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...