Two alternative approaches to access mixed hydride-amido zinc complexes: synthetic, structural and solution implications†‡
Abstract
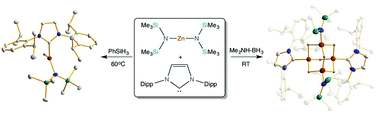
Using bis(amide) Zn(HMDS)2 (HMDS = 1,1,1,3,3,3-hexamethyldisilazide) as a precursor, this study explores the synthesis of N-heterocyclic carbene stabilized mixed amido-hydride zinc complexes using two alternative hydride sources, namely dimethylamine borane (DMAB) and phenylsilane PhSiH3. Hydride-rich zinc cluster Zn4(HMDS)2H6·2IPr (1) (IPr = 1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidene), which can be envisaged as a co-complex of IPr·ZnH2 and (HMDS)ZnH, is obtained when DMAB is employed, with the concomitant formation of heteroleptic bis(amido)borane [HB(NMe2)(HMDS)] and H2 evolution. NMR studies in d8-THF show that although the bulky carbene IPr does not bind to the zinc bis(amide), its presence in the reaction media is required in order to stabilise 1. Reactions using the slightly less sterically demanding NHC IXy (IXy = 1,3-bis-(2,6-dimethylphenyl)imidazol-2-ylidene) led to the isolation and structural elucidation of the carbene adduct Zn(HMDS)2·IXy (2). Contrastingly, mixtures of equimolar amounts of PhSiH3 and the zinc bis(amide) (60 °C, 3 h, hexane) afforded monomeric heteroleptic hydride (HMDS)ZnH·IPr (3). NMR studies, including DOSY experiments, revealed that while the integrity of 3 is retained in polar d8-THF solutions, in lower polarity C6D6 it displays a much more complex solution behaviour, being in equilibrium with the homoleptic species ZnH2·IPr, Zn(HMDS)2 and IPr.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...