Synthesis and Lewis acidity of fluorophosphonium cations†
Abstract
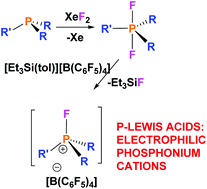
A series of fluorophosphonium salts, [R3PF][X] (R = alkyl or aryl; X = FB(C6F5)3, [B(C6F5)4]), have been prepared by reactions of phosphine/borane frustrated Lewis pairs (FLPs) with XeF2 or difluorophosphoranes with [Et3Si][B(C6F5)4]. As the substituents bound to phosphorus become increasingly electron withdrawing, the corresponding fluorophosphonium salts are shown to be increasingly Lewis acidic. Calculations were also performed to determine the relative fluoride ion affinities (FIA) of these fluorophosphonium cations.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...