Te–Te and Te–C bond cleavage reactions using a monovalent gallanediyl†
Abstract
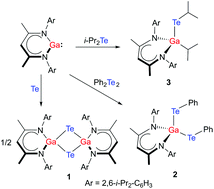
LGa (L = [(2,6-i-Pr2-C6H3)NC(Me)]2CH) reacts with elemental tellurium with formation of the Te-bridged compound [LGa-μ-Te]21, whereas the reactions with Ph2Te2 and i-Pr2Te occurred with cleavage of the Te–Te and Te–C bond, respectively, and subsequent formation of LGa(TePh)22 and LGa(i-Pr)Tei-Pr 3. 1–3 were characterized by heteronuclear NMR (1H, 13C, 125Te) and IR spectroscopy and their solid state structures were determined by single crystal X-ray analyses.

 Please wait while we load your content...
Please wait while we load your content...