Covalent attachment of diphosphine ligands to glassy carbon electrodes via Cu-catalyzed alkyne-azide cycloaddition. Metallation with Ni(ii)†
Abstract
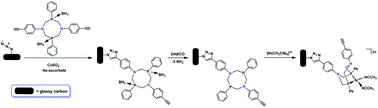
Covalent tethering of PPh2NC6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH2 ligands (PPh2NC6H4C
CH2 ligands (PPh2NC6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH2 = 1,5-di-(4-ethynylphenyl)-3,7-diphenyl-1,5-diaza-3,7-diphosphacyclooctane) to planar, azide-terminated glassy carbon electrode surfaces has been accomplished using a CuI-catalyzed alkyne-azide cycloaddition (CuAAC) coupling reaction, using a BH3←P protection-deprotection strategy. Deprotected, surface-confined ligands were metallated using [NiII(MeCN)6](BF4)2. X-ray photoelectron spectroscopic measurements demonstrate that metallation introduced 1.3 equivalents NiII per diphosphine onto the electrode surface. Exposure of the surface to a second diphosphine ligand, PPh2NPh2, resulted in the removal of Ni from the surface. Protection, coupling, deprotection, and metallation conditions were optimized using solution-phase model systems, with benzyl azide as a model for the azide-terminated carbon surface; these reactions generate a [NiII(diphosphine)2]2+ complex.
CH2 = 1,5-di-(4-ethynylphenyl)-3,7-diphenyl-1,5-diaza-3,7-diphosphacyclooctane) to planar, azide-terminated glassy carbon electrode surfaces has been accomplished using a CuI-catalyzed alkyne-azide cycloaddition (CuAAC) coupling reaction, using a BH3←P protection-deprotection strategy. Deprotected, surface-confined ligands were metallated using [NiII(MeCN)6](BF4)2. X-ray photoelectron spectroscopic measurements demonstrate that metallation introduced 1.3 equivalents NiII per diphosphine onto the electrode surface. Exposure of the surface to a second diphosphine ligand, PPh2NPh2, resulted in the removal of Ni from the surface. Protection, coupling, deprotection, and metallation conditions were optimized using solution-phase model systems, with benzyl azide as a model for the azide-terminated carbon surface; these reactions generate a [NiII(diphosphine)2]2+ complex.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...