Regioselective synthesis of highly functionalized alkenylboronates by Cu-catalyzed borylation of propargylic silylalkynes†
Abstract
High regioselectivity was achieved in the Cu(I)-catalyzed borylation of internal propargylic alkynes with a silyl substituent to afford multifunctionalized alkenylboron compounds. While both the silyl and propargylic substituents are known to act as directing groups, a N-heterocyclic carbene (NHC)–Cu complex furnished β-vinylboronate products (relative to Si) with high selectivity.
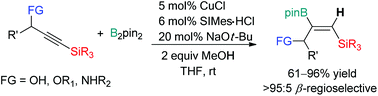
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...