Synthesis of cyclic polyesters: effects of alkoxy side chains in salicylaldiminato tin(ii) complexes†
Abstract
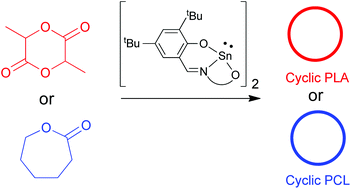
A new class of salicylaldiminato tin(II) catalysts having different alkoxy side chains has been developed. The ligands were modified to have different lengths and flexibilities such as –(CH2)2– (2a), –(CH2)3– (2b), –(ortho-C6H4)CH2– (2c) and –(CH2)2–O–(CH2)2– (2d). Complexes 2a, b were characterized crystallographically revealing a more constrained environment around the metal in complex 2a. These catalysts are active for the solvent-free polymerization of L-lactide and ε-caprolactone. Complex 2a having a shorter side chain was shown to better promote intramolecular transesterification affording cyclic polylactides and cyclic poly(ε-caprolactone). Complexes 2b and 2d having longer side chains produced cyclic poly(ε-caprolactone) as a major product but failed to give cyclic polylactides.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...