Reaction mechanism for the highly efficient catalytic decomposition of peroxynitrite by the amphipolar iron(iii) corrole 1-Fe†
Abstract
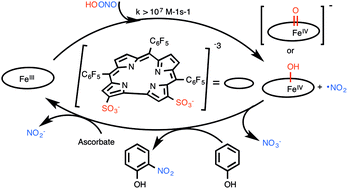
The amphipolar iron(III) corrole 1-Fe is one of the most efficient catalysts for the decomposition of peroxynitrite, the toxin involved in numerous diseases. This research focused on the mechanism of that reaction at physiological pH, where peroxynitrite is in equilibrium with its much more reactive conjugated acid, by focusing on the elementary steps involved in the catalytic cycle. Kinetic investigations uncovered the formation of a reaction intermediate in a process that is complete within a few milliseconds (k1 ∼ 3 × 107 M−1 s−1 at 5 °C, about 7 orders of magnitude larger than the first order rate constant for the non-catalyzed process). Multiple evidence points towards iron-catalyzed homolytic O–O bond cleavage to form nitrogen dioxide and hydroxo- or oxo-iron(IV) corrole. The iron(IV) intermediate was found to decay via multiple pathways that proceed at similar rates (k2 about 106 M−1 s−1): reaction with nitrogen dioxide to form nitrate, nitration of the corrole macrocyclic, and dimerization to binuclear iron(IV) corrole. Catalysis in the presence of substrates affects the decay of the iron intermediate by either oxidative nitration (phenolic substrates) or reduction (ascorbate). A large enough excess of ascorbate accelerates the catalytic decomposition of PN by 1-Fe by orders of magnitude, prevents other decay routes of the iron intermediate, and eliminates nitration products as well. This suggests that the beneficial effect of the iron corrole under the reducing conditions present in most biological media might be even larger than in the purely chemical system. The acquired mechanistic insight is of prime importance for the design of optimally acting catalysts for the fast and safe decomposition of reactive oxygen and nitrogen species.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...