Abstract
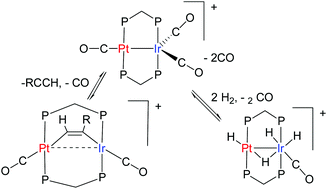
The chemistry of the heterobinuclear platinum–iridium complex [PtIr(CO)3(μ-dppm)2][PF6], 1, dppm = Ph2PCH2PPh2, is described. The reaction of a hydride with 1 gave [HPtIr(CO)2(μ-dppm)2], by displacement of the carbonyl ligand from platinum, while reaction of 1 with dihydrogen, hydrogen chloride or Ph2MeSiH gave the fluxional complex [PtIrH4(CO)(μ-dppm)2][PF6], [PtIrH2Cl2(CO)(μ-dppm)2][PF6], or [PtIrH(SiMePh2)(CO)2(μ-dppm)2][PF6], respectively, by oxidative addition at iridium. Complex 1 reacted, often regioselectively, with several alkynes to give the μ–η1,η1 bridging alkyne complexes [PtIr(μ-RCCR′)(CO)2(μ-dppm)2][PF6], R = H, R′ = Ph, 4-C6H4Me, CO2Me; R = Ph, R′ = CO2Me; R = R′ = CO2Me. The complex [PtIr(μ-HCC-4-C6H4Me)(CO)2(μ-dppm)2][PF6] reacted reversibly with CO to give [PtIr(μ-HCC-4-C6H4Me)(CO)3(μ-dppm)2][PF6] and [PtIr(CO)3(μ-dppm)2][PF6], 1. With HCl, [PtIr(μ-HCC-4-C6H4Me)(CO)2(μ-dppm)2][PF6] reacted to give [PtIrHCl(μ-HCC-4-C6H4Me)(CO)2(μ-dppm)2][PF6], by oxidative addition at iridium, and then the alkenylplatinum derivative [PtIrCl{HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(4-C6H4Me)}(CO)2(μ-dppm)2][PF6]. [PtIr(μ-HCC-4-C6H4Me)(CO)2(μ-dppm)2][PF6] reacted slowly with dihydrogen to give 4-MeC6H4CH
CH(4-C6H4Me)}(CO)2(μ-dppm)2][PF6]. [PtIr(μ-HCC-4-C6H4Me)(CO)2(μ-dppm)2][PF6] reacted slowly with dihydrogen to give 4-MeC6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and [PtIrH4(CO)(μ-dppm)2][PF6]. The complex [PtIr(μ-HCCPh)(CO)2(μ-dppm)2][PF6] is intensely luminescent in solution at room temperature, with features characteristic of a d8–d8 face-to-face complex.
CH2 and [PtIrH4(CO)(μ-dppm)2][PF6]. The complex [PtIr(μ-HCCPh)(CO)2(μ-dppm)2][PF6] is intensely luminescent in solution at room temperature, with features characteristic of a d8–d8 face-to-face complex.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...