Binding of an organo–osmium(ii) anticancer complex to guanine and cytosine on DNA revealed by electron-based dissociations in high resolution Top–Down FT-ICR mass spectrometry†
Abstract
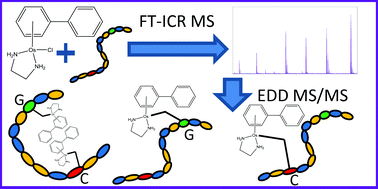
The OsII arene anticancer complex [(η6-bip)Os(en)Cl]+ (Os1-Cl; where bip = biphenyl, and en = ethylenediamine) binds strongly to DNA. Here we investigate reactions between Os1-Cl and the self-complementary 12-mer oligonucleotide 5′-TAGTAATTACTA-3′ (DNA12) using ultra high resolution Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS). Identification of the specific sites of DNA osmiation with {(η6-bip)Os(en)}2+ was made possible by the use of Electron Detachment Dissociation (EDD) which produced a wide range of assignable osmiated MS/MS fragments. In contrast, the more commonly used CAD and IRMPD techniques produced fragments which lose the bound osmium. These studies reveal that not only is guanine G3 a strong binding site for {(η6-bip)Os(en)}2+ but, unexpectedly, so too is cytosine C10. Interestingly, the G3/C10 di-osmiated adduct of DNA12 also formed readily but did not undergo such facile fragmentation by EDD, perhaps due to folding induced by van der Waal's interactions of the bound osmium arene species. These new insights into osmium arene DNA adducts should prove valuable for the design of new organometallic drugs and contribute to understanding the lack of cross resistance of this organometallic anticancer complex with cisplatin.
- This article is part of the themed collection: Metal Interactions with Nucleic Acids


 Please wait while we load your content...
Please wait while we load your content...