Three new solvent-directed Cd(ii)-based MOFs with unique luminescent properties and highly selective sensors for Cu2+ cations and nitrobenzene†
Abstract
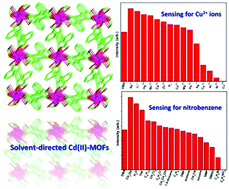
Three new solvent-induced metal–organic frameworks (MOFs)—[Cd(H2L)(H2O)3]·NMP (1), [Cd3(L)(H2O)4(OH)2] (2) and [Cd(L)0.5(H2O)]·H2O (3)—were designed and successfully prepared via solvothermal reaction by multidentate phenyltetracarboxylic acid [1,1′:4′,1′′-terphenyl]-2′,3,3′′,5′-tetracarboxylic acid (H4L) and Cd(II) salts in various solvent systems. Structural analyses indicated that the H2L/L ligands took three different coordination fashions in 1–3, and thus resulted in diversity of the targeted MOFs. Solid-state luminescent properties of the three MOFs were studied under ultraviolet light irradiation at ambient temperature; 3 in particular showed high selectivity and sensitivity for Cu2+ ions and nitrobenzene because of the quenching effect, which thus could make it a potential crystalline material for detecting these substances. The mechanisms of the quenching effect and sensing properties of 3 are discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...