Weak aurophilic interactions in a series of Au(iii) double salts†
Abstract
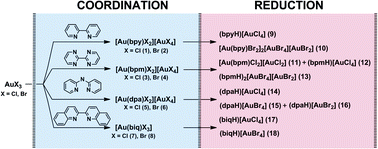
In this work, several new examples of rare AuIII⋯AuIII aurophilic contacts are reported. A series of gold(III) double salts and complexes, viz. [AuX2(L)][AuX4] (L = 2,2′-bipyridyl, X = Cl 1, Br 2; L = 2,2′-bipyrimidine, X = Cl 3, Br 4; L = 2,2′-dipyridylamine, X = Cl 5, Br 6), [AuX3(biq)] (biq = 2,2′-biquinoline, X = Cl 7, Br 8), [LH][AuX4] (L = 2,2′-bipyridyl, X = Cl 9; L = 2,2′-bipyrimidine, X = Cl 12; L = 2,2′-dipyridylamine, X = Cl 14, Br 15; L = 2,2′-biquinoline, X = Cl 17, Br 18), [AuBr2(bpy)]2[AuBr4][AuBr2] 10, [AuCl2(bpm)][AuCl2] 11, (bpmH)2[AuBr4][AuBr2] 13, and (dpaH)[AuBr2] 16 (1, 2, and 7 were reported earlier) was synthesized by coordination of a particular ligand to the AuIII center and subsequent reduction of the formed product with acetone. Inspection of the X-ray structural data for 1, 3–6, and 11 indicates that the AuIII metal centers approach each other closer than the sum of their van der Waals radii, thus forming the aurophilic contacts, which were confirmed by topological charge density analysis according to the Quantum Theory of Atoms in Molecules (QTAIM). In 1, 4, and 11, such contacts are located only between the metal centers of the ion pair, whereas in 3, the aurophilic interactions form the cation–anion–anion array, and in 5, the aurophilicity exists between the gold atoms of the cations. It was also demonstrated that the interatomic distance alone is not a reliable measure of the aurophilic interactions, at least at the weakest limit of the interaction strength, and it needs to be complemented with structural analysis of the whole molecule and computational results.

 Please wait while we load your content...
Please wait while we load your content...