Synthesis and the structure of 8-tetrahydrofuronium and 8-tetrahydropyronium derivatives of iron bis(dicarbollide)(-I) and their cleavage reactions†‡
Abstract
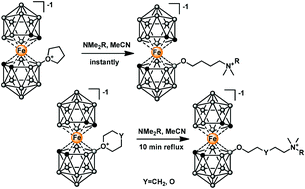
8-Tetrahydrofuronium and 8-tetrahydropyronium derivatives of iron bis(dicarbollide)(-I) were synthesized. Their reactions of ring cleavage by MeOH, N3−, amines and 1,2-bis(diphenylphosphino)ethane were investigated. 8-Tetrahydrofuronium iron bis(dicarbollide)(-I) was found to be more active in these reactions in comparison with 8-tetrahydropyronium and 8-dioxonium species, respectively. The first conjugates of iron bis(1,2-dicarbollide)(-I) with 2′-deoxyadenosine modified via the C-8 position of the purine base were synthesized. Reactions of these oxonium compounds with 1,2-bis(diphenylphosphino)ethane (dppe) led to zwitter-ionic monophosphonium salts. One of these compounds has given rise to a novel ferracarborane/cobaltacarborane hybrid complex.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...