Reactivity of ytterbium(ii) silylamide supported by a pyrrolyl–cyclopentadienyl ligand†
Abstract
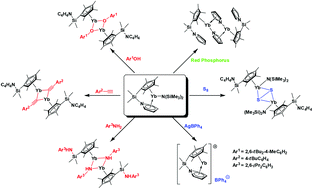
Reactivity of a divalent ytterbium amide LYbN(SiMe3)2 (1, L = Me4C5-SiMe2-NC4H4) supported by a pyrrolyl functionalized cyclopentadienyl ligand has been investigated. Reactions of 1 with the element sulfur led to the oxidation of the ytterbium to yield the disulfide-bridged ytterbium complex [LYbN(SiMe3)2]2(μ–η2:η2-S2) (2) while that with AgBPh4 resulted in the oxidation of the amide ligand to yield the divalent ytterbium complex LYb(BPh4) (3) along with the formation of silver and the N–N coupling product [N(SiMe3)2]2. Reactions of 1 with phenol, phenylacetylene and aniline led to the formation of the corresponding ytterbium aryloxide [LYb(μ-OAr1)]2 (4, Ar1 = 2,6-tBu2-4-MeC6H2), ytterbium alkynyl complex [LYb(μ-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CAr2)]2 (5, Ar2 = 4-tBuC6H4) and ytterbium amide [(Me4C5-SiMe2-NHAr3)Yb(μ-NHAr3)]2 (6, Ar3 = 2,6-iPr2C6H3), respectively. The structural analysis of the reaction products revealed that the silyl bridged pyrrolyl arm is not coordinated to the ytterbium center in compounds 2, 4 and 5. The formation of 6 involved the cleavage of the Si–Npyrrole bond, the same bond cleavage was also observed in the reaction of 1 with red phosphorus, leading to the pyrrolide bridged complex [LYb(μ–η1:η5-NC4H4)]2 (7).
CAr2)]2 (5, Ar2 = 4-tBuC6H4) and ytterbium amide [(Me4C5-SiMe2-NHAr3)Yb(μ-NHAr3)]2 (6, Ar3 = 2,6-iPr2C6H3), respectively. The structural analysis of the reaction products revealed that the silyl bridged pyrrolyl arm is not coordinated to the ytterbium center in compounds 2, 4 and 5. The formation of 6 involved the cleavage of the Si–Npyrrole bond, the same bond cleavage was also observed in the reaction of 1 with red phosphorus, leading to the pyrrolide bridged complex [LYb(μ–η1:η5-NC4H4)]2 (7).

 Please wait while we load your content...
Please wait while we load your content...