Reactions of heteroallenes with cyclam-based Zr(iv) complexes†
Abstract
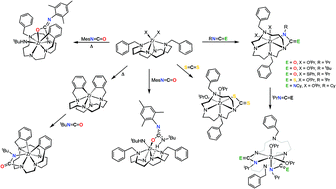
This work describes reactions of heteroallenes with diamido–diamine cyclam-based Zr(IV) complexes of the general formula (Bn2Cyclam)ZrX2 (X = OtBu, 1, OiPr, 2, SPh, 3, NHtBu, 14) as well as the di-orthometallated species ((C6H4CH2)2Cyclam)Zr, 17. The reactions of isocyanates or isothiocyanates with 1, 2 or 3 resulted in the formation of N-bonded ureate or thioureate cyclam complexes upon [2 + 2] cycloaddition of the Zr–Namido bonds of the cyclam to the heteroallene (4–9). DFT calculations showed that κ2-N,N′-ureate bonding is favoured over κ2-N,O-ureates, which in turn may be formed in reactions with bulky isocyanates as 1-naphthyl isocyanate (NpN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The reactions of 2 with N,N′-cyclohexylcarbodiimide (CyN
O). The reactions of 2 with N,N′-cyclohexylcarbodiimide (CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy) and carbon disulfide afforded guanidinate and dithiocarbamate fragments, respectively, appended to one of the nitrogen atoms of the cyclam ligand. These reactions represent a reliable method for the synthesis of asymmetrically N-functionalized cyclams giving rise to C1 symmetry Zr(IV) species by addition of one equivalent of heteroallenes. The reaction of (Bn2Cyclam)Zr(NHtBu)2, 14, with one equivalent of mesityl isocyanate (MesN
NCy) and carbon disulfide afforded guanidinate and dithiocarbamate fragments, respectively, appended to one of the nitrogen atoms of the cyclam ligand. These reactions represent a reliable method for the synthesis of asymmetrically N-functionalized cyclams giving rise to C1 symmetry Zr(IV) species by addition of one equivalent of heteroallenes. The reaction of (Bn2Cyclam)Zr(NHtBu)2, 14, with one equivalent of mesityl isocyanate (MesN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) also proceeds through insertion, involving one Zr–NHtBu bond. However, it was observed that the reaction of (Bn2Cyclam)Zr(NHtBu)2, 14, with MesN
O) also proceeds through insertion, involving one Zr–NHtBu bond. However, it was observed that the reaction of (Bn2Cyclam)Zr(NHtBu)2, 14, with MesN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O follows a different path if the reaction is carried out at 60 °C. In this case the reaction leads to [2 + 2] addition of the Zr–Ncyclam bond to the isocyanate, with a concomitant occurrence of orthometallation of the one benzyl pending group of the cyclam ring. The reaction of tBuN
O follows a different path if the reaction is carried out at 60 °C. In this case the reaction leads to [2 + 2] addition of the Zr–Ncyclam bond to the isocyanate, with a concomitant occurrence of orthometallation of the one benzyl pending group of the cyclam ring. The reaction of tBuN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with the di-orthometallated complex ((C6H4CH2)2Cyclam)Zr, 17, also gave a κ2-N,N′-ureate fragment, by isocyanate addition to the macrocycle. DFT calculations on these systems were conducted in an attempt to rationalise the reactivity patterns observed.
O with the di-orthometallated complex ((C6H4CH2)2Cyclam)Zr, 17, also gave a κ2-N,N′-ureate fragment, by isocyanate addition to the macrocycle. DFT calculations on these systems were conducted in an attempt to rationalise the reactivity patterns observed.


 Please wait while we load your content...
Please wait while we load your content...