A heterotrimetallic Ir(iii), Au(iii) and Pt(ii) complex incorporating cyclometallating bi- and tridentate ligands: simultaneous emission from different luminescent metal centres leads to broad-band light emission†
Abstract
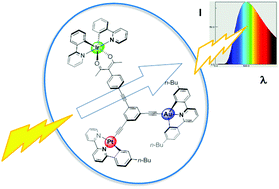
Di- and tri-nuclear metal complexes incorporating gold(III), iridium(III) and platinum(II) units linked via a 1,3,5-triethynylbenzene core are reported, together with the corresponding mononuclear complexes as models. The gold(III) and platinum(II) units comprise tridentate, cyclometallating, C^N^C and N^N^C-coordinating ligands, respectively, with the Ar–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– directly bound to the metal at the fourth coordination site. The iridium moiety is an Ir(ppy)2(acac) unit bound to the triethynylbenzene through a phenyl substituent at the 3-position of the acac ligand. The multinuclear compounds are prepared, using a modular synthetic strategy, from the monometallic complexes. All of the compounds are luminescent in solution at room temperature, and their photophysical properties were studied. The triplet excited state energies of the mononuclear complexes lie in the order Au > Ir > Pt. Consistent with this order, energy transfer from Au to Ir and from Au to Pt is observed, leading to quenching of the Au emission in the gold-containing multinuclear complexes. Energy transfer from Ir to Pt occurs at a rate that only partially quenches the Ir-based emission. As a result, the dinuclear Ir–Pt and trinuclear Au–Ir–Pt complexes display broad emission across most of the visible region of the spectrum.
C– directly bound to the metal at the fourth coordination site. The iridium moiety is an Ir(ppy)2(acac) unit bound to the triethynylbenzene through a phenyl substituent at the 3-position of the acac ligand. The multinuclear compounds are prepared, using a modular synthetic strategy, from the monometallic complexes. All of the compounds are luminescent in solution at room temperature, and their photophysical properties were studied. The triplet excited state energies of the mononuclear complexes lie in the order Au > Ir > Pt. Consistent with this order, energy transfer from Au to Ir and from Au to Pt is observed, leading to quenching of the Au emission in the gold-containing multinuclear complexes. Energy transfer from Ir to Pt occurs at a rate that only partially quenches the Ir-based emission. As a result, the dinuclear Ir–Pt and trinuclear Au–Ir–Pt complexes display broad emission across most of the visible region of the spectrum.
- This article is part of the themed collection: Luminescent Complexes and Materials for Light-Emitting Devices

 Please wait while we load your content...
Please wait while we load your content...