Geometric, electronic and magnetic structures of S = 19/2 and S = 20/2 thiophene-2-carboxylate functionalized Mn12 single molecule magnets†
Abstract
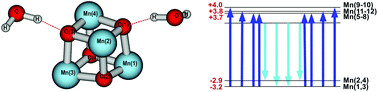
The geometric and magnetic structures of two structurally related, but magnetically inequivalent, single molecule magnets (SMMs) have been computationally characterized. The first SMM, with formula [Mn12O12(O2CC4H3S)16(H2O)2]−1 (I), has a half-integer spin (SI = 19/2) due to ferrimagnetic ordering. The second SMM, with formula Mn12O12(O2CC4H3S)16(H2O)4 (II), has an integer spin (SII = 20/2) and its geometric structure has been computationally predicted. Both SMMs include thiophene-2-carboxylate functional groups for potential use in molecular electronics. To determine structural and electronic differences between both SMMs, spin polarized density functional theory was applied to I and II. Hydrogen bonding of two and four Mn-bound water molecules in I and II, respectively, to thiophene-2-carboxylate oxygen atoms and inner cubane oxygen atoms is essential for structural stabilization of both complexes. The one-electron-reduction of I is concomitant with a structural asymmetry within its cubane whereby two ions, of nominal Mn4+(Si = 3/2) character, are inequivalent to the other two and acquire an incipient Mn3+(Si = 4/2) character. The geometric asymmetry in I provides an extra, albeit small, contribution to its zero field splitting and anisotropy barrier to spin reversal. Thus, despite its lower spin state, the anisotropy barrier of I is only slightly lower than that of II.

 Please wait while we load your content...
Please wait while we load your content...