Lithiated sulfoxides: α-sulfinyl functionalized carbanions†
Abstract
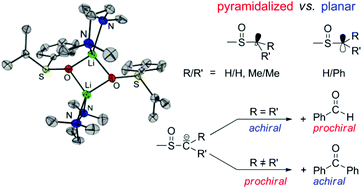
Reactions of alkyl aryl sulfoxides H–CRR′S(O)Ar with n-BuLi–TMEDA (TMEDA = N,N,N′,N′-tetramethylethylenediamine) afforded α-sulfinyl functionalized alkyl aryl lithium compounds of the type [Li2{CRR′S(O)Ar}2(TMEDA)2] (1, R/R′ = H/H, Ar = Ph; 2, R/R′ = H/H, Ar = p-Tol; 3, R/R′ = Me/Me, Ar = Ph; 4, R/R′ = H/Ph, Ar = Ph; 5, R/R′ = Me/Ph, Ar = Ph). The compounds were characterized by 1H, 13C and 7Li NMR spectroscopy and, except for 5, by single-crystal X-ray diffraction analyses. In crystals of 1, 2, 3 and 4·Et2O dinuclear molecules with four-membered Li2O2 rings were found. There are no Li⋯Cα contacts, thus, “free” carbanions are the main structural feature. Reactions of 1–6 (6, R/R′ = H/Me, Ar = Ph) with benzaldehyde and benzophenone afforded the corresponding sulfoxides of the type ArS(O)CRR′CHPhOH (1a–6a) and ArS(O)CRR′CPh2OH (1b–6b), respectively. The reactions yielding 1a/3a and 4b/6b proceeded with high diastereoselectivities. By X-ray diffractometry it has been shown that in the case of 3a and 4b the diastereomers consisting of the two enantiomers SSRC and RSSC were formed.

 Please wait while we load your content...
Please wait while we load your content...