Enlarging the tools for efficient enzymatic polycondensation: structural and catalytic features of cutinase 1 from Thermobifida cellulosilytica†
Abstract
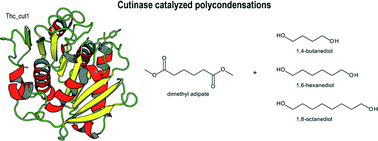
Cutinase 1 from Thermobifida cellulosilytica is reported for the first time as an efficient biocatalyst in polycondensation reactions. Under thin film conditions the covalently immobilized enzyme catalyzes the synthesis of oligoesters of dimetil adipate with different polyols leading to higher Mw (~1900) and Mn (~1000) if compared to lipase B from Candida antarctica or cutinase from Humicola insolens. Computational analysis discloses the structural features that make this enzyme readily accessible to substrates and optimally suited for covalent immobilization. As lipases and other cutinase enzymes, it presents hydrophobic superficial regions around the active site. However, molecular dynamics simulations indicate the absence of interfacial activation, similarly to what already documented for lipase B from Candida antarctica. Notably, cutinase from Humicola insolens displays a “breathing like” conformational movement, which modifies the accessibility of the active site. These observations stimulate wider experimental and bioinformatics studies aiming at a systematic comparison of functional differences between cutinases and lipases.

 Please wait while we load your content...
Please wait while we load your content...