New insights into the catalytic cleavage of the lignin β-O-4 linkage in multifunctional ionic liquid media†
Abstract
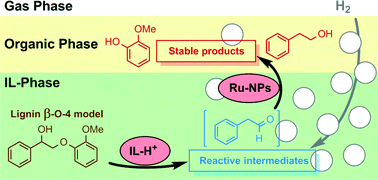
Ionic liquids are attractive reaction media for the solubilisation and depolymerisation of lignin into value-added products. However, mechanistic insight related to the cleavage of specific linkages relevant to efficient lignin depolymerisation in such solvents is still lacking. This study presents important insight into the scission of the most abundant lignin β-O-4 motif in Brønsted acidic ionic liquids. Using relevant model compounds, cleavage products were identified and undesired side reactions examined carefully. Stabilization of reactive intermediates was achieved in ionic liquids comprising both Brønsted acidic function and stabilized nanoparticles that comprise hydrogenation activity in order to suppress undesired side reactions. Especially, the in situ hydrogenation of the aldehyde intermediate originating from the acid-catalysed cleavage of lignin beta-O-4 model compounds into more stable alcohols was investigated. This is the first time that such products have been systematically targeted in these multifunctional reaction media in relation to lignin depolymerization.

 Please wait while we load your content...
Please wait while we load your content...