One-pot conversion of cephalosporin C by using an optimized two-enzyme process
Abstract
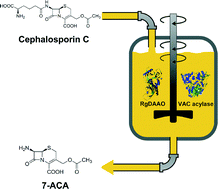
The main industrial process for producing 7-aminocephalosporanic acid (7-ACA), a precursor of semi-synthetic cephalosporin antibiotics, is the two-step enzymatic route based on D-amino acid oxidase and glutaryl acylase working in separate reactors. Taking advantage of the recently developed variants of cephalosporin C acylase from Pseudomonas N176 (named VAC) and the optimized recombinant overproduction of the two enzymes, we set up a one-pot system to directly convert cephalosporin C into 7-ACA. We optimized the process by identifying the most favorable operational conditions, substrate, and enzyme concentrations. Among the VAC variants employed, our results indicated that HS-HS-F72βR VAC is the best choice because of the high activity on both substrates (glutaryl-7-ACA and cephalosporin C) and the absence of substrate and product inhibition effects. Under optimized conditions and by adding further aliquots of the biocatalysts, >98% of cephalosporin C was converted, yielding 7-ACA as the main reaction product (oxo-7-ACA was below the detection level and glutaryl-7-ACA was <1 mM). At the 20 mL bioconversion scale, approximately 81 mg of 7-ACA are produced in 41 hours from 15 mM cephalosporin C. The low cost of the one-pot enzymatic production of 7-ACA is the main advantage of the proposed method, which is further strengthened by the good purity of the final product.

 Please wait while we load your content...
Please wait while we load your content...