Semimetal-functionalised polyoxovanadates
Abstract
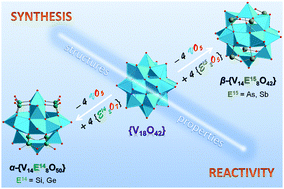
Polyoxovanadates (POVs), known for their wide applicability and relevance in chemical, physical and biological sciences, are a subclass of polyoxometalates and usually self-assemble in aqueous-phase, pH-controlled condensation reactions. Archetypical POVs such as the robust [VIV18O42]12− polyoxoanion can be structurally, electronically and magnetically altered by heavier group 14 and 15 elements to afford Si-, Ge-, As- or Sb-decorated POV structures (heteroPOVs). These main-group semimetals introduce specific chemically engineered functionalities which cause the generally hydrophilic heteroPOV compounds to exhibit interesting reactivity towards organic molecules, late transition metal and lanthanoid ions. The fully-oxidised (VV), mixed-valent (VV/VIV and VIV/VIII), “fully-reduced” (VIV) and “highly-reduced” (VIII) heteroPOVs possess a number of intriguing properties, ranging from catalytic to molecular magnet characteristics. Herein, we review key developments in the synthetic and structural chemistry as well as the reactivity of POVs functionalised with Si-, Ge-, As- or Sb-based heterogroups.


 Please wait while we load your content...
Please wait while we load your content...