Theoretical study of the coordination behavior of formate and formamidoximate with dioxovanadium(v) cation: implications for selectivity towards uranyl†
Abstract
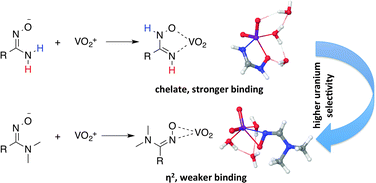
Poly(acrylamidoxime)-based fibers bearing random mixtures of carboxylate and amidoxime groups are the most widely utilized materials for extracting uranium from seawater. However, the competition between uranyl (UO22+) and vanadium ions poses a significant challenge to the industrial mining of uranium from seawater using the current generation of adsorbents. To design more selective adsorbents, a detailed understanding of how major competing ions interact with carboxylate and amidoxime ligands is required. In this work, we employ density functional theory (DFT) and wave-function methods to investigate potential binding motifs of the dioxovanadium ion, VO2+, with water, formate, and formamidoximate ligands. Employing higher level of theory calculations (CCSD(T)) resolve the existing controversy between the experimental results and previous DFT calculations for the structure of the hydrated VO2+ ion. Consistent with the EXAFS data, CCSD(T) calculations predict higher stability of the distorted octahedral geometry of VO2+(H2O)4 compared to the five-coordinate complex with a single water molecule in the second hydration shell, while all seven tested DFT methods yield the reverse stability of the two conformations. Analysis of the relative stabilities of formate–VO2+ complexes indicates that both monodentate and bidentate forms may coexist in thermodynamic equilibrium in solution. Investigations of VO2+ coordination with the formamidoximate anion has revealed the existence of seven possible binding motifs, four of which are within ∼4.0 kcal mol−1 of each other. Calculations establish that the most stable binding motif entails the coordination of oxime oxygen and amide nitrogen atoms via a tautomeric rearrangement of amidoxime to imino hydroxylamine. The difference in the most stable VO2+ and UO22+ binding conformation has important implications for the design of more selective UO22+ ligands.

 Please wait while we load your content...
Please wait while we load your content...