A sum-frequency generation spectroscopic study of the Gibbs analysis paradox: monolayer or sub-monolayer adsorption?†
Abstract
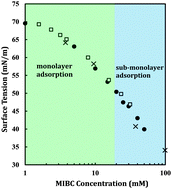
The Gibbs adsorption isotherm (GAI) has been considered as the foundation of surfactant adsorption studies for over a century; however, its application in determining the limiting surface excess has recently been intensively discussed, with contradictory experimental evidence either supporting or refuting the theory. The available arguments are based on monolayer adsorption models. In this paper, we experimentally and intellectually propose and validate the contribution of sub-monolayer adsorption to the GAI paradox. We utilize a powerful intrinsically surface-sensitive technique, vibrational sum-frequency generation spectroscopy (SFG), complementing with conventional tensiometric measurements to address these controversies both quantitatively and qualitatively. Our SFG results revealed that the precipitous decrease in surface tension directly corresponds to surface occupancy by adsorbates. In addition, the Gibbs analysis was successfully applied to the soluble monolayer of a surface-active alcohol to full saturation. However, the full saturation of the topmost monolayer does not necessarily mean that the surface adsorption was completed because the adsorption was observed to continuously occur in the sub-monolayer region soon after the topmost monolayer became saturated. Nonetheless, the Gibbs isotherm failed to account for the excess of alcohol adsorbed in this sub-monolayer region. This new concept of surface excess must therefore be treated thermodynamically.

 Please wait while we load your content...
Please wait while we load your content...