Unveiling the peculiar hydrogen bonding behavior of solvated N-heterocyclic carbenes†
Abstract
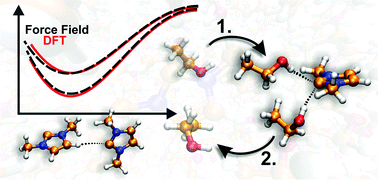
To investigate the hydrogen bonding and its dynamics of N-heterocyclic carbenes (NHCs) in solution, a molecular mechanical force field was fitted for the homologous series of 1,3-dialkylimidazol-2-ylidenes. During the exploration of the potential energy surface of the water/1,3-dimethylimidazol-2-ylidene system, it was observed for the first time that the carbene is prone to interaction with hydrogen bond donor molecules also from the rather unusual “on top” orientation, where the direction of the interplay is perpendicular to the plane of the NHC's ring. The fitting of the force field parameters for imidazol-2-ylidenes was found to be the best in the case of a two-site model, which reproduces not only the strength, but also the direction dependency of hydrogen bonding. With the aid of this tool, curious, hitherto unknown types of hydrogen bonding could be unveiled for NHCs. In the case of non-hydrogen bonding solvents, carbenes tend to form short lived, but structurally influental hydrogen bonds between each other via ring hydrogen atoms and the divalent carbon atoms. The chemically highly important hydrogen bond dynamics of NHCs was found to be facilitated by three center hydrogen bonding, where two alcohol molecules bind to a carbene, which is allowed only by the aforementioned relatively strong interaction between the NHC and the hydrogen bond donor in the “on top” orientation. The latter finding has significant effects on processes that involve this kind of replacement, such as the selective transesterification reactions, and the mechanism of proton exchange on azolium rings.

 Please wait while we load your content...
Please wait while we load your content...