Bifunctional Ce1−xEuxO2 (0 ≤ x ≤ 0.3) nanoparticles for photoluminescence and photocatalyst applications: an X-ray absorption spectroscopy study
Abstract
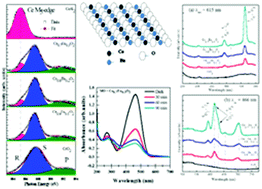
Ce1−xEuxO2 (0 ≤ x ≤ 0.3) nanoparticles (NPs) were synthesized by the chemical precipitation method. The microstructures and morphology were characterized by synchrotron X-ray diffraction and high resolution transmission electron microscopy. X-ray absorption near edge structure (XANES) spectra at the Eu M5,4-edge and atomic-multiplet calculations revealed that Eu3+ was predominantly present in the CeO2 lattice and Eu2+ was negligibly present within the entire doping range. The detailed analysis of the Ce M5,4-edge and the O K-edge has shown strong dependence of the Ce3+/Ce4+ ratio and oxygen vacancy with Eu content. Extended X-ray absorption fine structure (EXAFS) spectra at the Ce K-edge, along with theoretical fitting, have shown systematic variation in the coordination number, bond length and Debye–Waller factor with Eu doping. A blue shift in the absorption edge was observed which implies a net increase in the charge transfer gap between the O 2p and Ce 4f bands due to the increased number of Ce3+ ions in the Eu doped samples. The excitation and emission spectra of pure CeO2 NPs did not show any photoluminescence (PL) characteristic; however, Ce1−xEuxO2 (x = 0.1–0.3) NPs showed significant improvements in the 4f–4f, 5D0–7F2 and 5D0–7F1 transitions induced luminescence properties. Eu doping has two major effects on the electronic structure and optical properties of CeO2 NPs: the first, at an Eu content of 10 mol%, is the formation of Ce4+–O–Eu3+ networks, i.e., Eu3+ ions substitute the Ce4+ ions and introduce oxygen vacancies and Ce3+ ions in the host lattice, which favors the 5D0–7F2 induced PL properties. The other, at an Eu doping over 10 mol%, is the formation of both Ce4+–O–Eu3+ and Ce3+–O–Eu3+, i.e., Eu3+ ions not only take substitutional sites of Ce4+ ions but also replace a fraction of Ce3+ ions in the CeO2 lattice which favors 5D0–7F1 induced PL properties. As an application of CeO2 NPs towards the degradation of water pollutants, we demonstrated that the Ce1−xEuxO2 (0 ≤ x ≤ 0.3) NPs can serve as effective photocatalyst materials towards the degradation of the methyl-orange aqueous pollutant dye under UV light irradiation.

 Please wait while we load your content...
Please wait while we load your content...