Hydrogenation of Pt/TiO2{101} nanobelts: a driving force for the improvement of methanol catalysis
Abstract
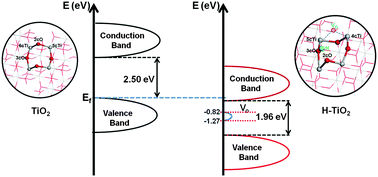
Single-crystalline anatase TiO2 nanobelts with a dominant surface of the {101} facet were hydrogenated and used as substrates of platinum for methanol oxidation reaction (MOR). The hydrogenated TiO2 anatase{101} supporting Pt exhibits a 228% increase of current density for methanol oxidation compared with the same system without hydrogenation under dark conditions. The synergetic interactions of hydrogenated anatase{101} with the Pt cluster were investigated through first principles calculations, and found that the hydrogenation shifts the conduction band minimum to the Fermi level of pristine TiO2, and reduces the activation barrier for methanol dissociation considerably. Thus, this work provides an experimental and theoretical basis for developing non-carbon substrates with high electro-catalytic activity toward MOR.

 Please wait while we load your content...
Please wait while we load your content...