Surface doping of La ions into ZnO nanocrystals to lower the optimal working temperature for HCHO sensing properties†
Abstract
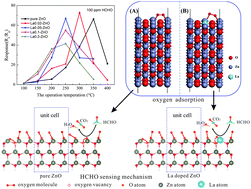
Lowering the working temperature without sacrificing other good gas-sensing properties is of particular interest to gas sensors for an excellent performance. In this work, La surface doped ZnO nanocrystals were successfully prepared by a facile thermal treatment with lanthanum nitrate (La(NO3)3) solution injected into ZnO thick films, which exhibited a remarkable decrease in the optimal working temperature for formaldehyde (HCHO) sensing properties. This was probably attributed to the formation of surface LaZn defects in the ZnO nanocrystals which was evidenced by XRD, XPS results and DFT calculations. The surface LaZn defects can introduce a shallower donor level than oxygen vacancies, and probably facilitate the charge transfer from oxygen species to ZnO for producing chemisorbed oxygen species more easily. This was in good agreement with the DFT results that the absorption energy of oxygen molecules on the surface of La doped ZnO was only −10.61 eV, much lower than that of pure ZnO. Moreover, the optimal working temperature of the La doped ZnO based sensor was significantly decreased from 350 to 250 °C without sacrificing the high and quick response to HCHO gas as the content of surface LaZn defects was increased gradually. Therefore, the behavior of the surface LaZn defects in the optimal working temperature revealed a HCHO response mechanism in ZnO, which can provide new insights into the enhanced HCHO sensing performance of gas sensors.


 Please wait while we load your content...
Please wait while we load your content...