The effect of surface modification by reduced graphene oxide on the electrocatalytic activity of nickel towards the hydrogen evolution reaction
Abstract
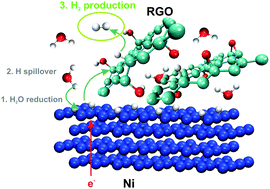
To find cheap, efficient and durable hydrogen evolution reaction catalysts is one of the major challenges when developing an alkaline water electrolysis system. In this paper we describe an electrochemically reduced graphene oxide (RGO)-modified Ni electrode, which could be used as a pre-eminent candidate for such a system. The experimentally determined characteristics of this electrode showing superior electrocatalytic activity were complemented by density functional theory calculations. Thermodynamic considerations led to the conclusion that H atoms, formed upon H2O discharge on Ni, spill onto the RGO, which serves as an H adatom acceptor, enabling continuous cleaning of Ni-active sites and an alternative pathway for H2 production. This mode of action is rendered by the unique reactivity of RGO, which arises due to the presence of O surface groups within the graphene structure. The significant electrocatalytic activity and life time (>35 days) of the RGO towards the HER under conditions of alkaline water electrolysis are demonstrated.

 Please wait while we load your content...
Please wait while we load your content...